- Debate
- Open access
- Published:
Screening: the information individuals need to support their decision: per protocol analysis is better than intention-to-treat analysis at quantifying potential benefits and harms of screening
BMC Medical Ethics volume 15, Article number: 28 (2014)
Abstract
Background
Providing individuals with the information necessary to make informed decisions is now considered an ethical standard for health systems and general practitioners.
Discussion
Results from intention-to-treat analysis have thus far been used to illustrate screening benefits and harms, but intention-to-treat analysis in most screening trials compares no intervention to invitation to screening. Therefore, the intervention arm includes everyone who was invited, regardless of actual participation. These results may be misleading for individual decision-making. We propose to use a per protocol analysis that includes all subjects who presented to screening and compares them to those in control arm, adjusting for self-selection bias. Such an analysis can give more accurate and useful information for individual decision-making.
Summary
Correct information for individual decision to participate in screening or not should consider the efficacy, benefits, and harms observed for subjects who actually participated at least once in screening compared to the control arm, adjusting for self-selection bias. Thus, per protocol analysis, even a very conservative one, should be used, not a full intention-to-treat analysis.
Background
Providing individuals with the information necessary to make informed decisions is now considered an ethical standard that screening programs and opportunistic secondary prevention must meet [1–3]. The general public must be aware of the benefits of screening, i.e. mortality reduction and, for cervical and colorectal cancer, reduced incidence, but also of its harms, i.e. overdiagnosis and unnecessary workup and treatment.
To this end, several systematic reviews have recently summarised the results of numerous randomised controlled trials (RCTs) on the benefits and harms of screening. These summaries provide pooled relative risk estimates as well as absolute numbers of events occurring in a cohort of screened individuals compared to what would occur in a cohort of unscreened individuals, based on intention-to-treat (ITT) analysis [4–6]. Presenting the results in terms of absolute numbers in a small cohort is the most effective way to provide correct information on the benefits and harms of a procedure: numerical information is exact, straightforward (thereby reducing cognitive complexity), and extremely concrete (thus easy to imagine) [7, 8]. However, these numbers, as we will see, may not be the most appropriate choice to support informed decision-making.
Most of the screening systematic reviews used ITT analysis to calculate the expected number of events (benefits and harms) in the screened and non-screened cohorts, which is a standard choice of analysis. For policy makers, intention-to-treat analysis - a weighted average of harms and benefits occurring in participants and non-participants alike - is the most useful since it measures the actual impact that a screening programme would have at the population level, taking into account also that the most important barrier to effectiveness is often non-participation [9, 10].
Most of the trials included in the systematic reviews that demonstrated screening efficacy (prostate, breast, and colorectal screening) were designed to randomise the target population to intervention (invitation to screening) or to no intervention (control – no invitation: for prostate cancer screening both PLCO [11] and ERSPC trials [12]; for breast cancer screening all the Swedish trials, Malmo, Goteborg, Stockholm, Two counties, the UK age trial, New York HIP trial, and Edinburgh. For references see the Cochrane systematic review [13] or the UK Independent Panel [3]; for colorectal cancer most of the trials on faecal occult blood test, Funen, Goteborg, Minnesota, and Nottingham. For references see the Cochrane systematic review [14]).
In the context of screening, however, intention-to-treat analysis results may not actually support individual decision-making. If the ITT analysis results from these RCTs are used to that end, we are providing the general public with information based on the comparison of the population randomised to receive the screening invitation with the population who did not receive the invitation. This information, in other words, examines the benefits and harms of screening between those who received the invitation and those who did not, and not the harms and benefits of actually participating in screening. The individual who must decide whether or not to participate in a screening programme, therefore, may be mislead by the very information intended to support him/her. This problem could be resolved by using a per protocol (PP) analysis, which compares those individuals who actually participated, i.e. at least presented for the first screening test, with those who were not invited. A recent review showed that most of the differences in the estimates of mortality reduction due to mammographic screening disappear when the figures are calculated only for women who were actually screened [15].
Discussion
Table 1 illustrates the kind of information provided to the general public on breast cancer screening: three plain language syntheses or fact boxes produced for individual decision-making on breast screening based on recent systematic reviews. These figures were produced by the UK Independent Panel [3], by the Euroscreen Working Group [4], and by the Harding Centre for Risk Literacy [5]. All three provide ITT estimates on the benefits of screening; surprisingly, the full papers by The UK Independent Panel and by Euroscreen also report per protocol estimates (women who actually responded). As for the harms of screening, the UK Independent Panel fact sheet clearly states that the estimate of overdiagnosis is calculated for women actually screened “as a proportion of cancers detected during their screening period”. Understanding the fact sheets is difficult, which defeats their purpose. Further, their plain language/narrative presentation does not clarify which estimates are used and to which population, invited or screened, they apply. Individuals may believe they have understood the information and have made an informed choice, but have they?
To illustrate the difference between intention-to-treat analysis results and per protocol estimates, we can use the same approach as the Harding Centre for Risk Literacy for mammography, applied to colorectal screening. With ITT, we obtain an estimate for colorectal cancer screening with faecal occult blood test of 85 deaths out of 10,000 persons screened for ten years and 100 deaths for the same population in the absence of screening. With a per protocol analysis, there would be 74 deaths out of 10,000 persons screened with at least one test in 10 years.
Which should the individual take into consideration in the decision-making process, 85 deaths per 10,000 screened or 74 deaths per 10,000 screened?
The Cochrane Review on colorectal screening does take these two calculations into account, however; both the mortality reduction for the whole randomised population and adjusted for participation are reported [14].
The first issue to be addressed concerns using ITT analysis results for the purpose of helping individuals invited to screening to decide whether or not to participate. Indeed, doing so would result in the paradox that the invitee would apparently be affected by any of the benefits and/or harms observed in the ITT analysis thanks solely to having received the invitation, regardless of actual participation. Clearly, however, both harms and benefits are downstreams of screening participation. Therefore, the correct numbers to use to help inform individual decision-making are those regarding the subjects who actually participated, which do not come from the ITT analysis but from PP.
Intention-to-treat remains the first and only analysis to test the hypothesis for the overall effectiveness of any intervention; rejection of the null hypothesis in the intention-to-treat analysis is a prerequisite to proceed to per protocol analysis.
In the case of a therapeutic intervention in the clinical setting, most of the causes of therapy interruption or non-compliance to the protocol are both prognostic factors and causally linked to the therapy itself (intolerance, side effects, etc.). The best prospective estimate of therapy efficacy that we can therefore give patients who must chose between therapeutic options is based on intention-to-treat analysis, given that it cannot be known a priori whether a therapy will be tolerated and/ or finished.
An individual’s decision to participate in screening, however, is not usually determined by potential negative or positive prognostic factors, and there is no causal link between participation and screening results. Therefore, if participants and non-participants differ in their baseline mortality or incidence this will be due to a self-selection bias.
Further, the target screening population is healthy; in the absence of other interventions, it is thus absolutely unlikely that a large number of deaths or cancer diagnoses will occur in the time elapsing between invitation and participation. Therefore, any potential bias that per protocol analysis may introduce due to the exclusion of outcomes that occurred between randomization and screening test should not be relevant, while it can be quite relevant when the mortality rate in the study population is high, i.e. in many therapeutic trials [16].
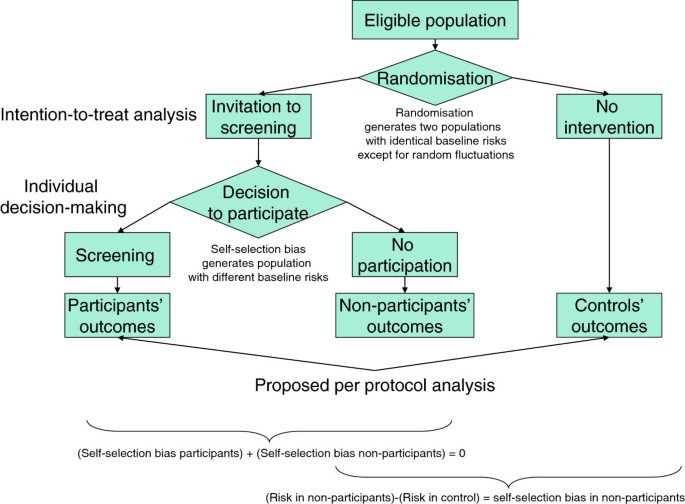
Given the absence of any causal link between participation and screening results (the decision is made before the first screening test) and given the data produced by the trials, the self-selection bias mentioned above can be adjusted for. In fact, we can measure incidence and mortality in the controls and compare them with those of the non-participants, thereby obtaining a direct measure of the self-selection bias (Figure 1). This measure is based on the outcomes themselves and thus includes all the possible effects of detected and undetected confounding variables.
In conclusion, a correct per protocol analysis can produce data useful to support individual decision-making providing it meets the following conditions: 1) it must include all the randomised subjects who presented for the test; 2) it must not exclude any subject for any reason after presentation; 3) it must compare the results in this cohort with those of the control arm; 4) it must appropriately adjust for self-selection bias. The conceptual flow chart of this strategy is represented in Figure 1.
A similar framework could be applied to estimate benefits and harms of other preventive interventions in which trials are designed to measure the impact on population while the impact on individuals depends on participation in the intervention itself.
Summary
Providing individuals with the information necessary to make informed decisions is now considered an ethical standard for health systems and general practitioners.
Results from intention-to-treat analysis have thus far been used to illustrate screening benefits and harms.
Intention-to-treat analysis in most screening trials compares no invitation (control) to invitation to screening (intervention). The intervention arm therefore includes everyone who was invited, regardless of actual participation. These results may be misleading for individual decision-making.
Correct information should consider the efficacy observed for subjects that participated at least once in screening compared to the control arm.
Abbreviations
- ITT:
-
Intention to treat
- PP:
-
Per protocol.
References
Austoker J: Gaining informed consent for screening. Is difficult—but many misconceptions need to be undone. BMJ. 1999, 319: 722-723. 10.1136/bmj.319.7212.722.
Giordano L, Webster P, Segnan N, Austoker J: Guidance On Breast Screening Communication. European Guidelines For Quality Assurance In Breast Cancer Screening And Diagnosis. Edited by: Perry N, Broeders M, de Wolf C. 2006, Luxembourg: European Communities, 379-394. 4
Austoker J, Giordano L, Hewitson P, Villain P: Communication. European guidelines for quality assurance in colorectal cancer screening and diagnosis. Edited by: Segnan N, Patnick J, von Karsa L. 2010, Luxembourg: European Communities, 301-339. 1
EUROSCREEN Working Group: Summary of evidence of breast cancer service screening outcomes in Europe and first estimates of benefit and harm balance sheet. J Med Screen. 2012, 19 (Suppl. 1): 5-13.
Independent UK Panel on Breast Cancer Screening: The benefits and harms of breast cancer screening: an independent review. Lancet. 2012, 17;380 (9855): 1778-1786.
Hardin centre for Health Litteracy: Fact boxes. (last access 30/08/2013).http://www.harding-center.com/index.php/en/what-you-should-know/facts-boxes,
Giordano L, Cogo C, Patnik J, Paci E, Euroscreen Working Group: Communicating the balance sheet in breast cancer screening. J Med Screen. 2012, 19 (Suppl. 1): 67-71.
Timmermans DR, Ockhuysen-Vermey CF, Henneman L: Presenting health risk information in different formats: the effect on participants’ cognitive and emotional evaluation and decisions. Patient Educ Couns. 2008, 73: 443-447. 10.1016/j.pec.2008.07.013.
Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J: The determinants of screening uptake and interventions for increasing uptake: a systematic review. Health Technol Assess. 2000, 4: i-vii. 1-133
Ferroni E, Camilloni L, Jimenez B, Furnari G, Borgia P, Guasticchi G, Giorgi Rossi P: Working group methods to increase participation: how to increase uptake in oncologic screening: a systematic review of studies comparing population-based screening programs and spontaneous access. Prev Med. 2012, 55: 587-596. 10.1016/j.ypmed.2012.10.007.
Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD, PLCO Project Team: Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009, 360 (13): 1310-1319. 10.1056/NEJMoa0810696.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A, ERSPC Investigators: Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009, 360: 1320-1328. 10.1056/NEJMoa0810084.
Gøtzsche PC, Jørgensen KJ: Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013, 6: CD001877-doi: 10.1002/14651858.CD001877.pub5. Review. PubMed PMID: 23737396
Hewitson P, Glasziou P, Irwig L, Towler B, Watson E: Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev. 2007, CD001216-Review. PubMed PMID: 17253456, 1
Duffy SW, Hsiu-Hsi Chen T, Smith RA, Ming-Fang Yen A, Tabar L: Real and artificial controversies in breast cancer screening. Breast Cancer Manage. 2013, 2: 519-528. 10.2217/bmt.13.53.
Montori VM, Guyatt GH: Intention-to-treat principle. CMAJ. 2001, 165: 1339-1341.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6939/15/28/prepub
Acknowledgements
I want to thank Jacqueline Costa for assistance in the English editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declares that he has no competing interests. Non financial competing interests: the author is involved in several projects to increase appropriateness in diagnostic test use and in evaluating the impact of organised screening programs.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Giorgi Rossi, P. Screening: the information individuals need to support their decision: per protocol analysis is better than intention-to-treat analysis at quantifying potential benefits and harms of screening. BMC Med Ethics 15, 28 (2014). https://doi.org/10.1186/1472-6939-15-28
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6939-15-28